Cyto Conference 2024
Edinburgh International Convention Center 150 Morrison St, Edinburgh, United KingdomJoin Discovery Life Sciences at Cyto Conference 2024 May 4-8, 2024 | Edinburgh International Convention Center, Edinburgh, Scotland
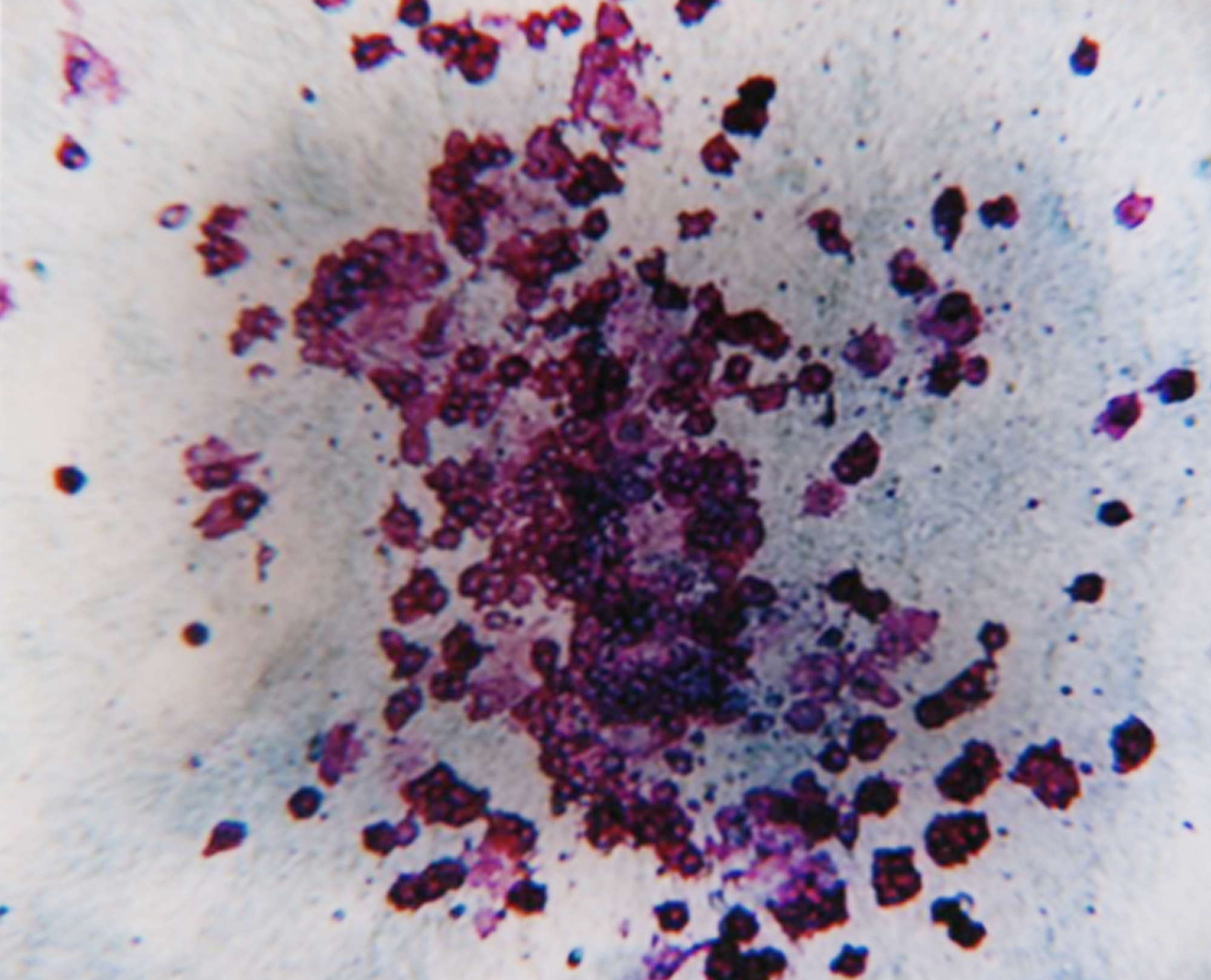
Assess drug efficacy against blood cancer progenitor cells.

We incubate diseased cells with your test compound(s) in a semi-solid, methylcellulose-based medium and we then assess cultures for changes in the number of cancer progenitor-derived colonies. We can also test your drug in a standard CFC assay to look for off-target toxicity to healthy donor progenitor cells.
Advantages:
The preclinical screening method that helps clients eliminate bad players earlier in the drug development process. White Paper
Colony Forming Cell assays can predict compound induced toxicities including neutropenia, thrombocytopenia, anemia and lymphotoxicity. Video
This in vitro assay has been validated by ECVAM (European Centre for the Validation of Alternative Methods) and correlates well with clinical neutropenia for many drug classes. Protocol

Join Discovery Life Sciences at Cyto Conference 2024 May 4-8, 2024 | Edinburgh International Convention Center, Edinburgh, Scotland
Join Discovery Life Sciences at APT 2024 May 14-16, 2024 | Merck Research Laboratories, Boston, Massachusetts
Join Discovery Life Sciences at ISCT 2024 Stop by our booth #453 or schedule a meeting with our CGT team. May 28- 31, 2024 | Vancouver, Canada
Copyright © 2024 Discovery Life Sciences. All rights reserved.
Designed & Developed by Altitude Marketing